COINFYCARE EL02C Manual - Página 2
Navegue en línea o descargue pdf Manual para Equipamiento médico COINFYCARE EL02C. COINFYCARE EL02C 6 páginas. Electrical cardiology table
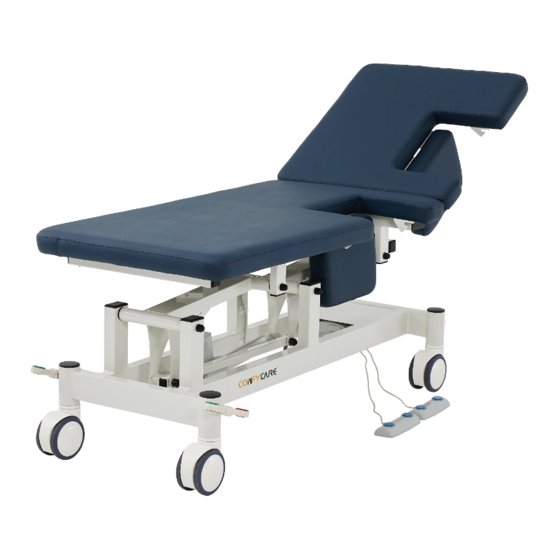
SAFETY PRECAUTIONS
1. During the adjustment of the table, do not put any
part of the body into the gap of any moving parts.
2. Please operate with both hands when adjusting the
leaning angle of back, foot or head sections.
3. When the patient is on the table, it is not recommended
to adjust the angle of the table top.
4. Before using the product, ensure the four feet of
the table frame touch the ground at the same time and
check the stability of the table frame.
5. Before connecting to the power supply, please check
whether the incoming line to the controller is intact.
6. A regular inspection (at least once a year) is needed
for the treatment table.
7. Do not exceed the table weight capacity of 250 kg.
The safe working load of the arm rest is 15 kg.
8. Always clean the table after each use with a soft cloth
dampened with water and a mild antibacterial detergent.
Do not use abrasive cleaners..
9. Disconnect the table from the power source before
attempting any maintenance, installation, removal or
replacement procedures to prevent electrical shock and
possible damage to the table.
10. Please DO NOT make any changes to the table.
Replacement parts or labor can be provided by
Coinfycare, the selling dealer or a service technician
certified by the manufacturer.
CONTENTS OF CARTON
Electrical Cardiology Table: 1 unit
Instruction Manual: 1 unit
NORMAL OPERATING CONDITIONS
Temperature: 5 ℃ - 40 ℃
Relative humidity: 30% - 75%
Air pressure range: 860-1060hpa
Power supply: AC 220V ± 10%, 50Hz
REGULATION AND PRODUCT
STANDARDS
This table complies with the European regulation of
medical devices (EU) 2017/745 and with the following
standards:
- EN 60601-1:2006+A1:2013+AC:2014
Medical electrical equipment. Part 1: General
Requirements for Basic Safety and Essential
Performance.
- IEC/EN 60601-2-52:2009+A1:2015 clause
201.9.8.3.2
Medical electrical equipment. Part 2-52: Particular
requirements for basic safety and essential
operation of medical tables.
- EN 60601-1-6:2010+A1:2015
Medical electrical equipment. Part 1-6: General
Requirements for basic safety and essential
performance. Collateral standard: Suitability for
use
- EN 60601-1-2:2015
Medical electrical equipment. Part 1-2: General
requirements for basic safety and essential
performance. Collateral standard:
Electromagnetic disturbances. Requirements and
tests.
- EN ISO 13485:2016
Medical devices - Quality management systems -
Requirements for regulatory purposes
(ISO 13485:2016)
DIN EN ISO 13485:2016
PRECAUTIONS AND SAFETY
INDICATIONS
Coinfycare is not liable for any material or personal
damage due to misuse or incorrect use of this table
or for any purpose other than described in this user
manual.
Inspect the condition of the table before using it for
the first time and on a recurring basis as indicated in
the "MAINTENANCE" section.
Keep table out of high moisture environments.
Do not transport the table over rough or outdoor
surfaces. The retractable caster system could
become damaged and inoperable.
This table should be operated, transported, and
stored in temperatures between 5° C to + 45° C,
with max humidity of 75%.
DO NOT operate this table in an environment
where other devices are being used that
intentionally radiate electromagnetic energy in an
unshielded manner. Portable and mobile
communications equipment can affect Medical
Electrical Equipment.
