iHealth BP7 소유자 매뉴얼 - 페이지 12
{카테고리_이름} iHealth BP7에 대한 소유자 매뉴얼을 온라인으로 검색하거나 PDF를 다운로드하세요. iHealth BP7 16 페이지. Wrist
iHealth BP7에 대해서도 마찬가지입니다: 빠른 시작 매뉴얼 (4 페이지), 소유자 매뉴얼 (16 페이지)
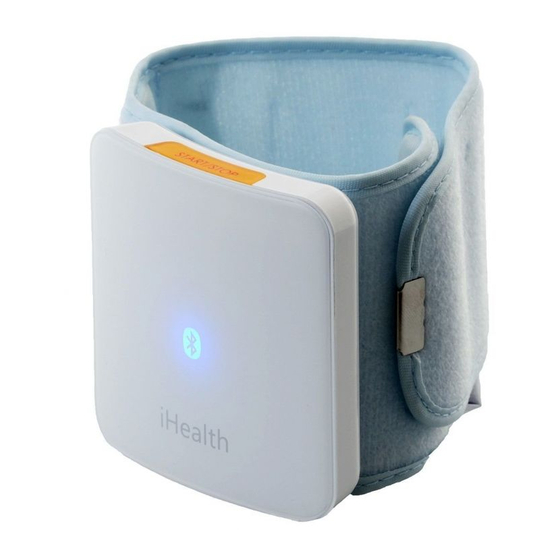
Lotus Global Co., Ltd.
15 Alexandra Road, London UK, NW8 0DP
EC
REP
Tel: +0044-20-75868010 Fax: +0044-20-79006187
ANDON HEALTH CO., LTD.
No. 3 Jinping Street, Ya An Road, Nankai District, Tianjin 300190,
China
Tel: 86-22-60526161
IMPORTANT INFORMATION REQUIRED BY THE FCC
This device complies with Part 15 of the FCC Rules. Its operation is subject to
the following two conditions:
(1)This device may not cause harmful interference, and
(2)this device must accept any interference received, including interference
that may cause undesired operation.
Changes or modifications not expressly approved by iHealth Lab Inc. would void
the user's authority to operate the product.
Note: This product has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference in a residential
installation. This product generates, uses, and can radiate radio frequency energy and,
if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this product does cause
harmful interference to radio or television reception, which can be determined by
turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
—Consult the dealer or an experienced radio/TV technician for help.
This product complies with Industry Canada. IC: RSS-210
IC NOTICE
This device complies with Industry Canada licence-exempt RSS standard(s).
Operation is subject to the following two conditions:
(1) this device may not cause interference, and
(2) this device must accept any interference, including interference that may
cause undesired operation of the device.
This product is approved in accordance to R&TTE directive transmitter.
OTHER STANDARDS AND COMPLIANCES
The Wireless Blood Pressure Wrist Monitor corresponds to the following
standards:
12
