4M Green Science Potato Clock Manual
Browse online or download pdf Manual for Science Education products 4M Green Science Potato Clock. 4M Green Science Potato Clock 2 pages.
Also for 4M Green Science Potato Clock: Manual (11 pages)
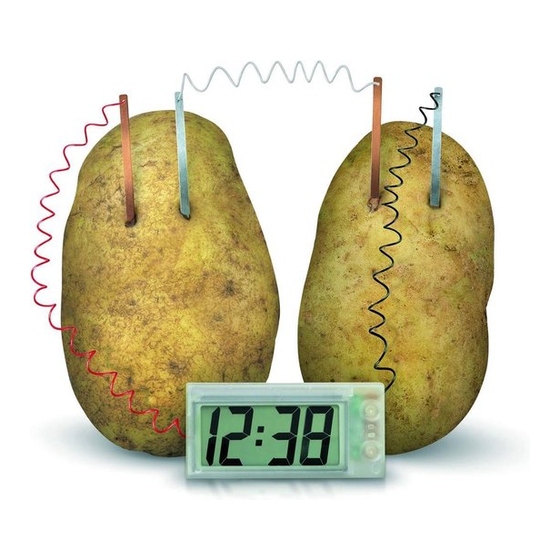
G . F u R T H E R E X P E R I M E N T S
Put some soft drink into the pots provided. Insert the
copper and zinc plates into the pots, as shown in the
diagram, taking care that the metal plates do not touch
each other. The clock should now start to work. You may
experiment with different liquid like salt water, fruit
juices; or fruit like lemon, orange, tomato etc. The fun
is unlimited.
H . F u N F A C T S
• The copper and zinc strips are called electrodes, and the potato is called an electrolyte.
• The potato battery works in the same way as the batteries used in electrical and electronic devices, such as torches,
radios and MP3 players. These batteries all contain different chemicals that produce electricity.
• Fruit and vegetables work well too. They contain plenty of particles that allow current to flow between the metal
strips.
• Battery types are named after the chemicals used inside them. Common types are zinc-carbon, nickel metal hydride
(NiMH), nickel cadmium (Ni-Cad).
• The chemicals in a battery are used up as the battery provides electricity. When no chemicals are left, the battery
is dead.
• Some batteries can be recharged when they are dead. Feeding electricity into a rechargeable battery reverses the
chemical changes inside the battery that happen when it produces electricity.
• The first battery was made by Italian scientist Alessandro Volta (1745-1827). He built a pile of metal discs with
card soaked in salty water between them. It produced a small electric current. The battery is now known as a Voltaic
pile.
• A fuel cell is a special type of battery. It produces electricity by the reaction between two chemicals. For example,
a hydrogen fuel cell produces electricity from the reaction between hydrogen and oxygen, which produces water. The
chemicals are constantly fed into the cell, so it never runs out.
• A non-rechargeable battery can't be recharged. Never try!
• Batteries contain some dangerous chemicals. Never open them up or cut them open, and always try to dispose of
them properly at a recycling centre.
• Copper is a very good conductor of electricity. It is used to make wires and cables.
• Zinc is used to galvanise steel objects such as garden tools and screws. The objects are coated with zinc, which
protects the steel from rusting.
I. QUESTION AND COMMENTS
We treasure you as a customer and your satisfaction with this product is important to us. In case you have any
comments or questions, or you find any parts of this kit missing or defective, please do not hesitate to contact
our distributor in your country, whose address is printed on the package. You are also welcome to contact our
marketing support team at Email: [email protected], Fax (852) 25911566, Tel (852) 28936241, Web site:
WWW.4M-IND.COM
COPYRIGHT 2008 4M INDuSTRIAL DEVELOPMENT LIMITED
A . S A F E T Y M E S S A G E S
To Parents: Read all instructions before providing
guidance to your children.
1. Please read through these instructions before you start.
2. Adult supervision and assistance are required at all times.
3. Intended for children of ages 8 and up.
4. This kit and its finished product contain small parts which may cause choking if misused. Keep away from
children under 3 years old.
5. Metal parts may have sharp edges. Adult assistance is required when assembling these parts.
6. Do not connect any of the parts provided to any AC wall socket or any batteries. This may cause electric shock or
a short circuit.
7. The LCD watch may temporary lose its function at electrostatic discharge environment, but it resumes its normal
function by resetting the device.
B . C O N T E N T S
Digital clock with wires
Transparent tapes
WARNING:
CHOCKING HAZARD
- Small parts.
Not for Children under 3 years.
Copper and zinc strips
Pots
Connecting Wire
