4M Green Science Potato Clock Посібник - Сторінка 8
Переглянути онлайн або завантажити pdf Посібник для Годинник 4M Green Science Potato Clock. 4M Green Science Potato Clock 11 сторінок.
Також для 4M Green Science Potato Clock: Посібник (2 сторінок)
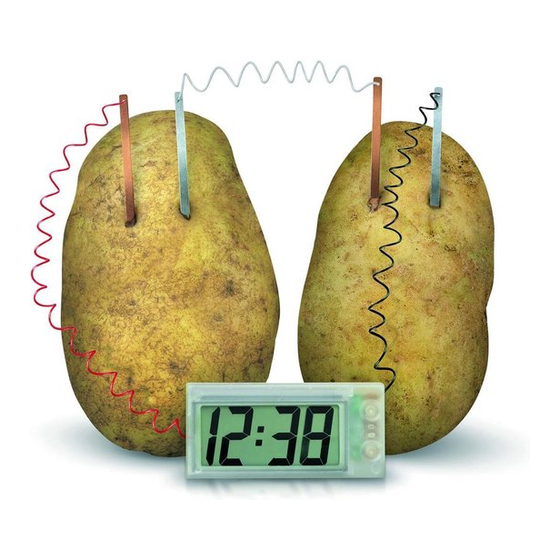
G. F U R T H E R E X P E R I M E N T S
Put some soft drink into the pots provided. Insert the
copper and zinc plates into the pots, as shown in the
diagram , taking care tha t the metal plates do not touch
each other. Th e clock should now start to work. You may
experiment with different liquids like salt water, fruit
juices; or fruit like lemon, orange, tom ato etc. The fun
is unlimited.
H. F U N F A C T S
• The copper and zinc strips are called electrodes, and the potato is called an electrolyte.
• The potato battery works in the same way as the batteries used in electrical and electronic devices, such as torches,
radios and M P3 players. These batteries all contain different chemicals th a t produce electricity.
• Fruit and vegetables work well too. They contain plenty of particles tha t allow current to flow between the metal
strips.
• Battery types are named after the chemicals used inside th e m . Common types are zinc-carbon, nickel metal hydride
(N iM H ), nickel cadm ium (N i-C a d ).
• The chemicals in a battery are used up as the battery provides electricity. When no chemicals are left, th e battery
is dead.
• Some batteries can be recharged when they are dead. Feeding electricity into a rechargeable battery reverses the
chemical changes inside the battery th a t happen when it produces electricity.
• The first battery was made by Italian scientist Alessandro Volta (1 7 4 5 -1 8 2 7 ) . He built a pile of metal discs with
card soaked in salty water between them . It produced a sm all electric current. The battery is now known as a Voltaic
pile.
• A fuel cell is a special type of battery. It produces electricity by the reaction between tw o chemicals. For exam ple,
a hydrogen fuel cell produces electricity from the reaction between hydrogen and oxygen, which produces w ater. The
chemicals are constantly fed into the cell, so it never runs out.
• A non-rechargeable battery can't be recharged. Never try!
• Batteries contain some dangerous chemicals. Never open them up or cut them open, and always try to dispose of
them properly at a recycling centre.
• Copper is a very good conductor of electricity. It is used to make wires and cables.
• Zin c is used to galvanise steel objects such as garden tools and screws. The objects are coated with zin c , which
protects the steel from rusting.
I. Q U ES TIO N AND C O M M EN TS
We treasure you as a custom er and your satisfaction w ith this product is important to u s . In case you have any
comments or questions, or you find any parts of this kit missing or defective, please do not hesitate to contact
our distributor in your country, whose address is printed on the package. You are also welcome to contact our
m arketing support team a t Em a il: in fodesk@ 4M -IN D.com , Fax (852) 2 5 9 115 6 6 , Tel (852) 2893S241, W eb site:
W W W .4M -IND.COM
