ATYS MEDICAL Microflow-S Kullanıcı Dokümantasyonu - Sayfa 2
Kişisel Bakım Ürünleri ATYS MEDICAL Microflow-S için çevrimiçi göz atın veya pdf Kullanıcı Dokümantasyonu indirin. ATYS MEDICAL Microflow-S 12 sayfaları.
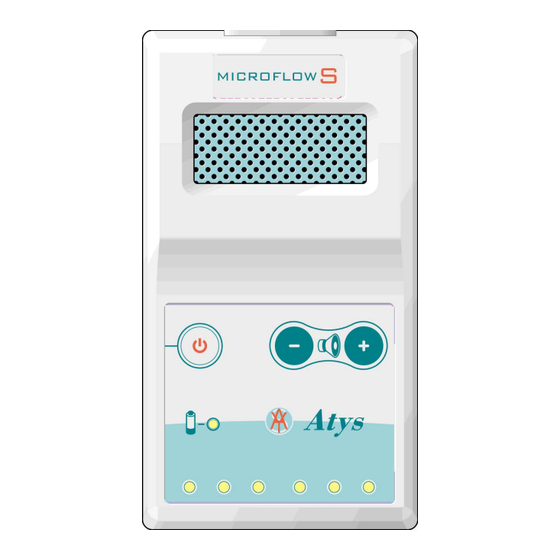
This document contains all information on the Atys MICROFLOW S devices manufactured by Atys.
Information in this document is subject to change without notice and does not represent a commitment on the
part of Atys.
Atys does not assume liability for damages that may occur because of the use of the information in this
manual.
No part of this manual may be reproduced or transmitted in any form for any purpose other than the use of the
purchaser of Atys MICROFLOW S.
1
Symbols ........................................................................................................................................................ 3
1.1
Device................................................................................................................................................... 3
1.2
Packaging ............................................................................................................................................. 3
2
Models & accessories................................................................................................................................... 4
3
Application specification ............................................................................................................................... 4
3.1
Intended medical indication .................................................................................................................. 4
3.2
Intended patient population .................................................................................................................. 4
3.3
Intended part of the body or type of tissue applied to or interacted with.............................................. 4
3.4
Intended user profile............................................................................................................................. 4
3.5
Intended conditions of use.................................................................................................................... 4
3.6
Expected service life............................................................................................................................. 4
3.7
Contact duration on applied parts and accessible parts ...................................................................... 4
3.8
Contraindication.................................................................................................................................... 4
3.9
Essential performance.......................................................................................................................... 5
4
CAUTION...................................................................................................................................................... 5
4.1
Operator................................................................................................................................................ 5
4.2
Storage environment ............................................................................................................................ 5
4.3
Operating environment ......................................................................................................................... 5
4.4
Electrical safety .................................................................................................................................... 5
4.5
Maintenance and service...................................................................................................................... 6
4.6
Ultrasound field..................................................................................................................................... 6
4.7
Environmental protection...................................................................................................................... 6
4.8
Electromagnetic compatibility ............................................................................................................... 6
5
Standard and regulation ............................................................................................................................... 7
5.1
Quality management ............................................................................................................................ 7
5.2
Regulation............................................................................................................................................. 7
5.3
Safety and performance ....................................................................................................................... 7
5.4
Ultrasound ............................................................................................................................................ 7
5.5
Usability ................................................................................................................................................ 7
5.6
Risk management................................................................................................................................. 7
5.7
Electromagnetic compatibility ............................................................................................................... 7
6
Environmental data....................................................................................................................................... 8
6.1
Battery .................................................................................................................................................. 8
6.2
Physical specifications.......................................................................................................................... 8
6.3
Doppler sound output ........................................................................................................................... 8
7
Operating ...................................................................................................................................................... 9
7.1
Theory of operation .............................................................................................................................. 9
7.2
Device description ................................................................................................................................ 9
7.3
CLEANING ........................................................................................................................................... 9
7.4
BATTERY REPLACEMENT ............................................................................................................... 10
8
Service........................................................................................................................................................ 10
8.1
Microflow S spare parts ...................................................................................................................... 10
8.2
Probe warranty ................................................................................................................................... 10
8.3
Assembly ............................................................................................................................................ 10
8.4
Test..................................................................................................................................................... 11
8.5
Probe connector wiring....................................................................................................................... 11
9
Document revision history .......................................................................................................................... 11
20 174 C
Microflow-S pocket Doppler
User's Documentation
Page 2/12
